Water solubility
Water solubility is an important parameter, which gives information on the polarity of the molecule and its capacity to be extracted by an aqueous solvent or to form “hydrogen bond” interactions.
As this parameter is very well documented for most molecules, it is easy to find the solubility of the solute to be extracted. It depends on temperature: the higher the temperature, the more soluble the compound will be in water.
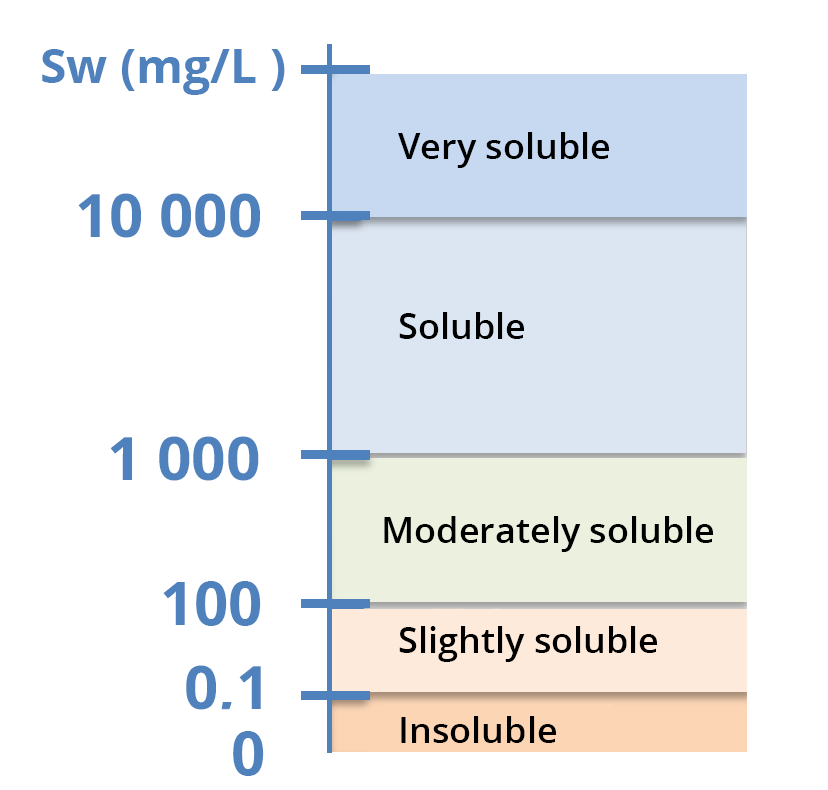
Solubility is expressed in g/L (soluble to very soluble compounds in water), mg/L (moderate solubility) or µg/L (very low solubility) – see solubility scale.