Determination of dissolved oxygen
When chemical equilibrium is reached, and according to Henry’s law, the solubility of O2 gas depends on the partial pressure of O2 above the solution.
pO2 = xO2L . KH,O2
where xO2L is the O2 mol fraction in water, and KH,O2 the Henry’s law constant of the gas.
Henry’s equilibrium is not verified in environments where O2 gas is dissolved and used continuously (biological or chemical systems): this is the case for rivers and waste water. The dissolved dioxygen concentration is below the expected concentration, which is evidence of pollution.
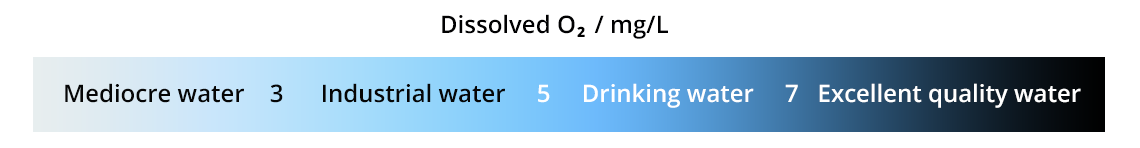
To determine O2 concentration in water, several methods are possible:
- By colorimetric titration and the Winkler method.
- With the use of a Clark cell.
Click on each box for more information