Analysis of a spring water
Determination of NO3- ions by colorimetric titration
Dosing principle
This method of colorimetric determination of nitrate ions can be applied to NO3- concentrations between 0.15 and 15 mg.L-1.
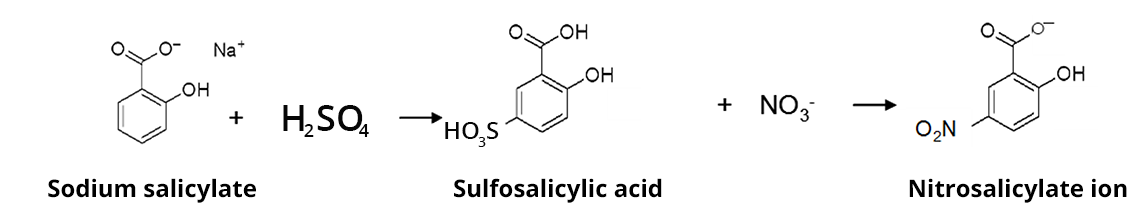
In an anhydrous medium, sulfosalicylic acid, created upon reaction of sulfuric acid with sodium salicylate, reacts with nitrates to form a mix of ortho- and para- sodium nitrosalicylate. In a basic environment, the nitrosalicylate anion is relased and its stable yellow color allows for a colorimetric titration at wavelength 415 nm.
The addition of sodium and potassium double tartrate together with sodium hydroxide prevents the precipitation of calcium and magnesium salts.