Experiment A: influence of volatility
Collect the liquids below in vials:
- toluene
- acetone
- ethanol
- dimethylformamide
- dichloromethane
Using capillaries, make the deposits of the previous liquids (by separating each deposit by about 1 cm). Observe IMMEDIATELY under a UV lamp, then again a few minutes later. Conclude.
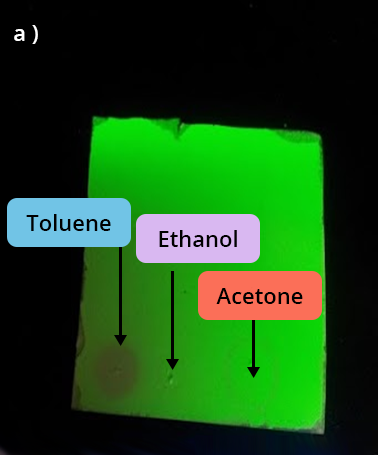
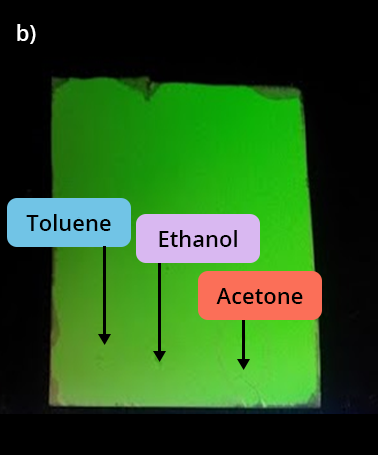
Observation under UV lamp of 3 solvent deposits:
Toluene – Ethanol - Acetonea) Right after the deposit
b) 1 min after the deposit

We observe that:
- toluene and acetone are volatile since they are no longer revealed a minute after the deposit
- ethanol does not absorb under UV.
Click on each box to see the result