How to perform a TLC?
Polarity of the phases
ELUENT POLARITY
It deeply affects its eluting power, namely its ability to drive polar compounds.
There is a classification of solvents according to their polarity (eluotropic series).
ADSORBENT POLARITY
The adsorbents employed in chromatography can be sorted according to their interaction forces with polar compounds.
In TLC, the stationary phase is usually made of silica (for organic compounds), possibly alumina or otherwise cellulose (for very polar compounds).
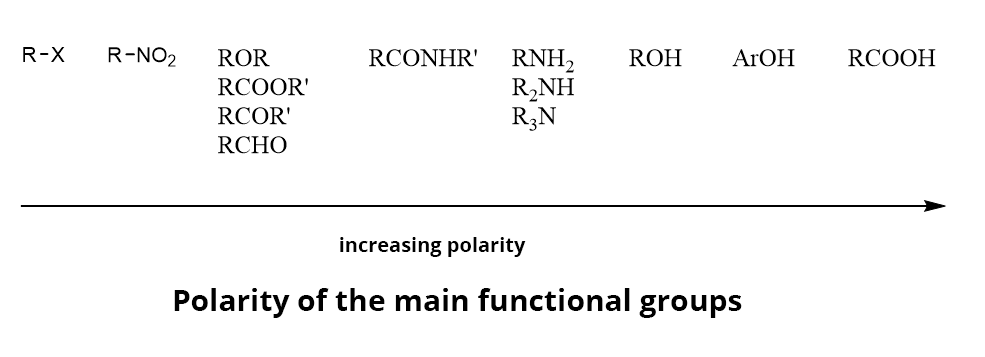
ORGANIC ANALYTE POLARITY
The main chemical functions can be sorted according to their polarity.
Consequences:
Relative migration sequence of organic compounds:
In normal phase adsorption chromatography, the stationary phase is more polar than the mobile one. Thus low-polar solutes, like aliphatic hydrocarbons, are only slightly adsorbed while the polar solutes are strongly adsorbed.
As a result, low-polar compounds (like aromatic hydrocarbons) will then migrate faster than polar compounds: their retardation factor will be higher.
Eluent choice: it is crucial because it allows for the fine adjustment of the differential migration rates of compounds. To this end, solvent mixtures can be used.