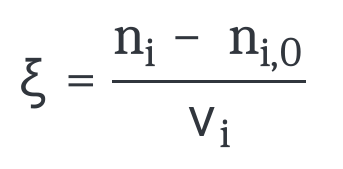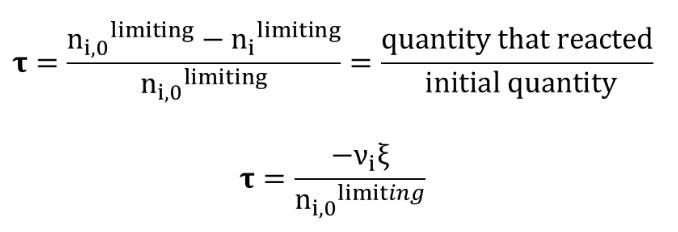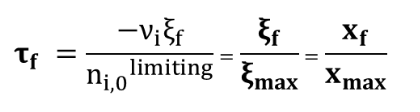The titration reaction
In the case of titration, the experimenter seeks a reaction between two chemicals: the titrand (R, Ctitrant) whose concentration is known, and the analyte whose concentration is unknown (T, Ctitré).
The titration reaction can be written this way:
ν1T + ν2R →
titration products
In equilibrium, quantitative and total reactions:
Chemical reactions are in equilibrium if all reagents and products of the reaction coexist and chemical equilibrium is achieved.
The final extent ξf is therefore far from reaching its maximal value ξmax
K° values are therefore higher (respectively lower) than the unit if the balance is in favor of the products (respectively the reagents). If K° is equal to 1, the reaction is neither in favor of the reagents nor the products.
A reaction is total if a total consumption of the reagents is observed, which means the maximal extent ξmax has been reached. The final extent rate τf equals 1. More precisely, considering a total reaction, the system reaches its full term.
A reaction is called quantitative if the equilibrium is strongly in favor of the products, without necessarily reaching the maximum extent ξmax. The final extent rate τf is below 1 but close to 1 (≈ 0,95 - 1).
Extent rate for a chemical reaction
The extent rate is defined in terms of the limiting reagent as follows:
with :
n
i,0 : initial amount of substance (t = 0)
n
i : amount of substance at time t
v
i : stoichiometric factor for the limiting reagent.
ξ : extent of the reaction
The
final extent rate at τf is such that:
The extent rate is dimensionless and is particularly interesting when the reaction is in stoichiometric proportions. Then, the extent rate is the same for all reagents.