Measurement guide
Calibration
1) Select calibration mode.
2) Select the correct temperature for the buffers if an automatic temperature correction is not performed.
3) Prepare the buffer solutions intended for the calibration by pouring a sufficient volume of the solution into clean containers.
4) Rinse the electrode with distilled or demineralized water.
 Wipe with soft paper without rubbing.
Wipe with soft paper without rubbing.
5) Take the first buffer solution, stir gently and immerse the electrode. Keep the electrolyte fill port open during measurement.
 Be sure to use the buffer solutions in the correct order for the calibration.
Be sure to use the buffer solutions in the correct order for the calibration.
Press the calibration button (or its equivalent) on the pH meter.
6) Wait for the measurement to stabilize.
7) Remove the electrode from the buffer solution and rinse it.
8) Take the second buffer solution, stir gently and immerse the electrode.
9) Press the calibration button (or its equivalent) on the pH meter.
10) Wait for the measurement to stabilize.
A 2 or 3 point calibration can be carried out.
11) If the calibration is correct, press OK and choose pH mode.
2) Select the correct temperature for the buffers if an automatic temperature correction is not performed.
3) Prepare the buffer solutions intended for the calibration by pouring a sufficient volume of the solution into clean containers.
4) Rinse the electrode with distilled or demineralized water.
 Wipe with soft paper without rubbing.
Wipe with soft paper without rubbing.5) Take the first buffer solution, stir gently and immerse the electrode. Keep the electrolyte fill port open during measurement.
 Be sure to use the buffer solutions in the correct order for the calibration.
Be sure to use the buffer solutions in the correct order for the calibration.Press the calibration button (or its equivalent) on the pH meter.
6) Wait for the measurement to stabilize.
7) Remove the electrode from the buffer solution and rinse it.
8) Take the second buffer solution, stir gently and immerse the electrode.
9) Press the calibration button (or its equivalent) on the pH meter.
10) Wait for the measurement to stabilize.
A 2 or 3 point calibration can be carried out.
11) If the calibration is correct, press OK and choose pH mode.
What is the calibration for?
The calibration is necessary in order to adjust the zero and the slope of the straight line E(mv) = f(pH) according to Nernst’s equation.
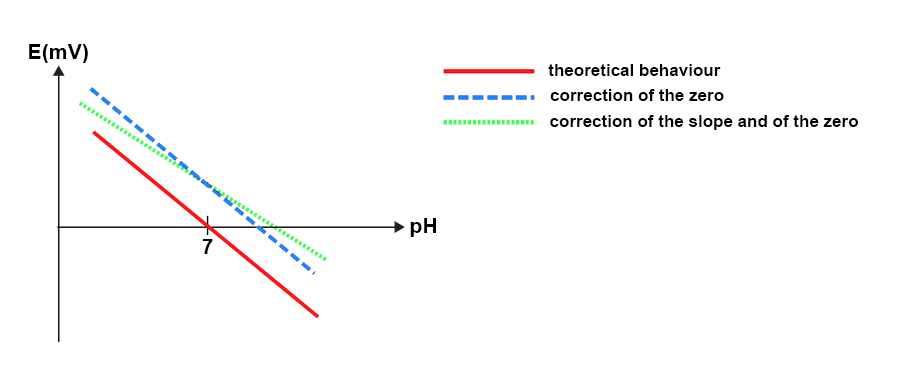
E = E0 + 2.3RT / nF * log [H3O+]
0 mV ~ pH 7
Slope: 2.3RT / nF
Incorrect calibration if:
- Slope
- Zero offset by +/- 30 mV
Cause of miscalibration
Causes
- Impure calibration buffer (contaminated, use-by date expired).
Test with new buffer solutions.
- Electrode:
Check the mV signal with a fresh buffer solution at pH 7.
Electrolyte: Check for…
Sufficient electrolyte level.
Absence of electrolyte crystals.
Electrolyte use-by date.
Diaphragm:
Check for the absence of air bubbles at the level of the diaphragm.
Clogging: immerse the liquid junction in a pH 4 solution overnight.
Membrane: Check its physical state.
If membrane dirty, degrease with ethanol, acetone or soapy water. Then rehydrate the membrane by immersing in an acid solution (0.1 M HCl).
If measuring a protein-rich solution: remove deposits by dipping the electrode bulb in 5% pepsin solution in 0.1 M HCl.