1) Pour a sufficient volume of the sample solution into a clean container so that the liquid junction of the electrode (diaphragm) is immersed in the sample.
2) Make sure that the sample’s temperature is known or that it is measured during the determination of the pH by an integrated or separate thermal sensor.
4) Gently stir the sample and immerse the pH electrode in the solution.
5) Press the pH meter’s measurement button and wait for a stable output value.
6) Remove the electrode from the solution and rinse with distilled or demineralized water.
Effect on the dissociation of the molecule.
Effect of temperature on the pH measurement
- Effect on the molecule’s dissociation
HA + H2O ⇔ H3O+ + A-
Equilibrium defined by the dissociation constant

Which is temperature-dependent according to Van’t Hoff’s law

 It is not possible to correct these deviations so it is important to note the temperature.
It is not possible to correct these deviations so it is important to note the temperature.
- Effect on electrode response:
According to Nernst’s equation, the slope of the response depends on the temperature:
E = E0 + 2.3RT / nF * log [H3O+]
The effect of temperature can thus be compensated for by correcting the pH value by the effective slope of the line.
 It is important to perform the calibration at the same temperature as the measurement.
It is important to perform the calibration at the same temperature as the measurement.
 Electrodes have maximum operating manipulation temperatures!!!
Electrodes have maximum operating manipulation temperatures!!!
Effect of ionic strength on pH measurement
In the Nernst equation, more than the concentration, it is the activity of the ion (a) that needs to be included in the pH calculation:
pH = - log a
H3O+
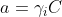
with
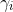
the activity coefficient
Debye Hückel’s law: 
With α the diameter of the hydrated ion (900pm for H3O+)
I the ionic force 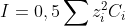
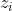
the charge of the present ions,
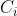
the concentration
pH measurement in non-aqueous media
In organic media or non-aqueous solutions (less than 5% water) the classical definition of pH does not apply. The conventional pH range from 0 to 14 based on the dissociation of water is no longer valid.
Example of the pH range in solvents:
In a non-aqueous solvent: measurement of a relative pH value rather than an absolute value.
In order to make quantitative measures in non-aqueous solvents: establish a calibration curve of the pH glass-electrode with different samples of known composition that meet the conditions prevailing in the samples to be measured.
 In the presence of solvent, the electrode’s membrane is deteriorated: rehydrate the gel layer in a solution rich in ions between the experiments.
In the presence of solvent, the electrode’s membrane is deteriorated: rehydrate the gel layer in a solution rich in ions between the experiments.
Electrode diaphragm clogging: Cause
Presence of proteins, elements rich in fat.
Precipitation of the electrolyte (KCl, AgCl) present.
KCl: precipitates in the presence of Hg 2+, Ag+, Pb 2+, ClO4-.
AgCl precipitates in the presence of Br-, Cn-, I- , sulfur derivatives, cystine and cysteine.
Electrode diaphragm clogging: Solution
Clogging by silver sulfide (Ag2S)
Clean with a solution of 8% of thiourea in 0.1 mol/L HCl.
Clogging by silver chloride (AgCl)
Soak the electrode in a concentrated ammonia solution.
Clogging by proteins
Immerse the electrode for several hours in a pepsin/HCI solution (5% pepsin in 0.1 mol/L HCl).
Other types of clogging
Clean the electrode in an ultrasonic bath with water or a solution of 0.1 mol/L HCl.
 Before taking any measurements, check that the calibration has been done.
Before taking any measurements, check that the calibration has been done. 









