Determination of Cl- ions by colorimetric titration: the Fajans method
Dosing principle
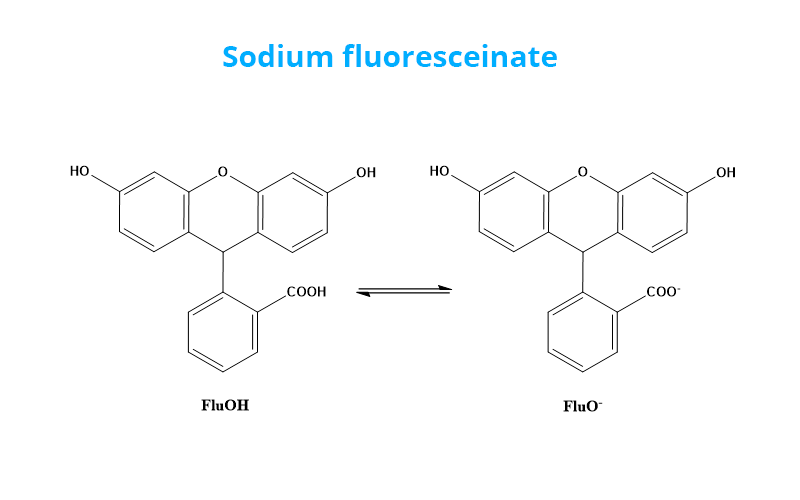
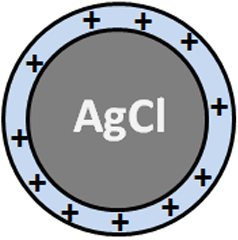
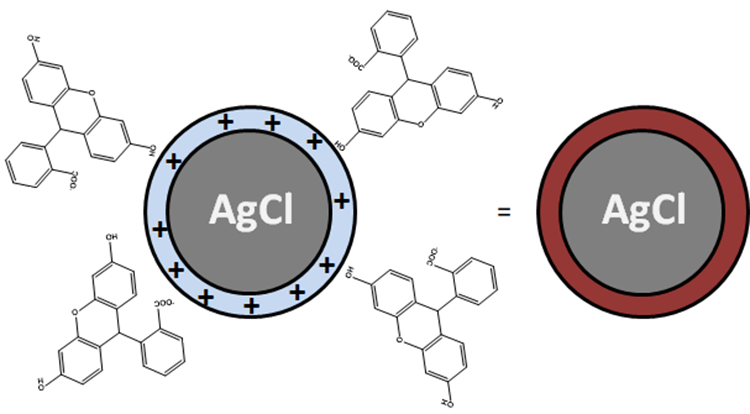
The determination of Cl- concentration is based on the titration of Cl- ions by adding AgNO3 to the water with sodium fluoresceinate (FluoNa). A white solid of AgCl is formed (1) before equivalence point. At the equivalence point, the solution takes on an orange color characteristic of the adsorption of Fluo- at the surface of the AgCl particles (2). Indeed, before the equivalence point, Cl- ions are in excess in the environment so that the surface the formed AgCl particles is negatively charged. After equivalence, the Ag+ ions are in excess in the environment so that the surface of the formed AgCl particles is positively charged (1’). Fluo- can then adsorb on the surface of the particles to form AgCl(s)-Fluo(ads)*.
(1) Ag+(aq) + Cl-(aq) = AgCl(s) (surface charged negatively)
(1’) AgCl(s) (surface charged negatively) + Ag+ = AgCl(s) (surface charged positively) + Ag+
(2) AgCl(s) (surface charged positively) = AgCl(s)-Fluo(ads)
(1’) AgCl(s) (surface charged negatively) + Ag+ = AgCl(s) (surface charged positively) + Ag+
(2) AgCl(s) (surface charged positively) = AgCl(s)-Fluo(ads)
Data:
pKs(AgCl) = 9.8; pkA (FluOH/FluO-) = 6.4.
*Ag-Fluo(s) does not precipitate because the FluO- concentration is too low to reach the solubility limit.