Common difficulties: tips and tricks
How can you make a deposit if the sample to be analyzed is not soluble in the eluent?
A solution can be made in a solvent other than the eluent. However, in order to avoid a different local migration of the sample, it is necessary to dry the plate before eluting.
How do you know if enough has been deposited?
If the product absorbs under UV, it is sufficient, before elution, to check under a UV lamp whether or not a deposit is observed. If this is not the case, the solution deposited is then too low in concentration.

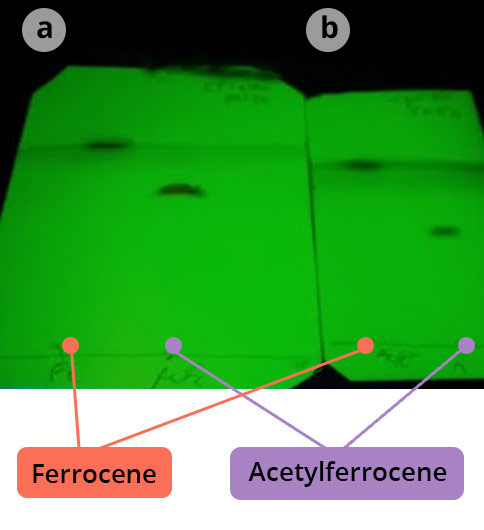
Why make co-deposits?
As a general rule, the mixture to be analyzed, the starting reagents, the pure products if they are commercial and the co-deposits (analysis mixture + reagents or commercial products) are deposited.

What should be done if compounds leave trails on the TLC?
This phenomenon can be due to the presence of chemical functions establishing strong hydrogen bonds with silica. The compounds then tend to trail on the stationary phase, the spot losing its disc shape and becoming very elongated; the determination of a frontal ratio is then very difficult.
Examples:
- RCO2H
- use an eluent containing an organic acid (AcOH, HCO2H) at a low %
- Amine
- Add a protic solvent (EtOH)
- Or add a base (Et3N) up to a few %