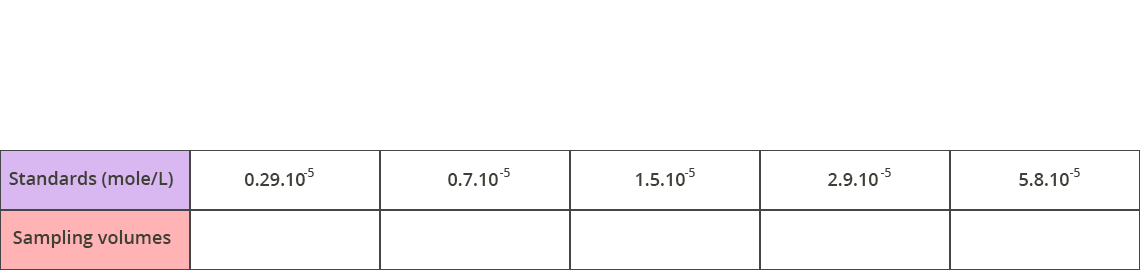
Exercise 1: Calculation of the sampling volumes for the calibration
The aim is to carry out an external calibration in order to measure the concentration of a dye in wastewater treated by chemical oxidation, so as to assess the residual amount of dye remaining in the treated water – this will ultimately allow us to estimate the effectiveness of the oxidation treatment in degrading this dye.
In order to do this, a dye stock solution must be prepared beforehand; the standard solutions of the calibration will be prepared from this stock solution.
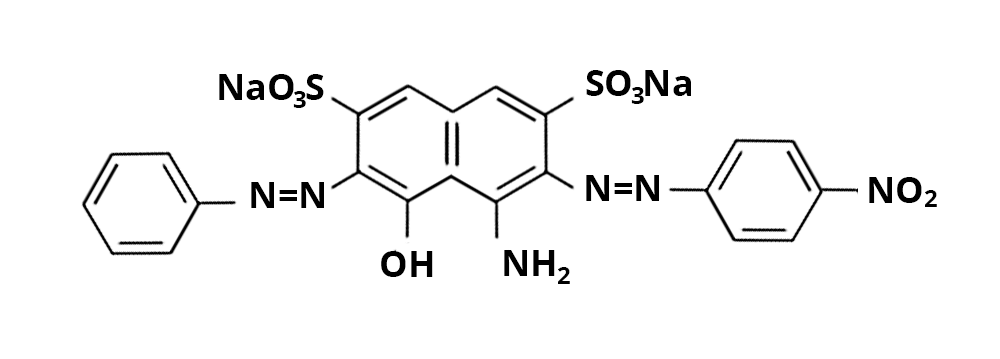
The dye in question (Acid Black 1) is a toxic diazo dye with a molar mass of 616.5 g/mol – its formula is given below:
A stock solution of this dye must be prepared at 2.9 x10-4 mol/L: a 1 liter flask is available for this.

50 mL volumetric flasks are available for preparing standard solutions with the following concentrations: 0.29 x 10-5 mol/L – 0.7 x 10-5 mol/L – 1.5 x 10-5 mol/L – 2.9 x 10-5 mol/L – 5.8 x 10-5 mol/L.