What should you do if the sample produces a higher signal than the maximum signal of the calibration range considered?
Dilute the sample before taking the measurement If the dilution is properly carried out, a sample with an analyte concentration within the range of concentrations of the calibration can be obtained.
Prepare a more suitable calibration range This solution could also be considered provided that the concentrations of the analyte to be determined are not too high so as not to risk saturating the signal (linear range not respected) or contaminating the measuring instrument by the excessively concentrated analyte.
Prepare a new sample In the same conditions, the result will be identical!
What should you do if the sample produces a signal that is too weak for the calibration range?
Concentrate the sample before taking the measurement This solution requires special attention in order not to lose the analyte, especially by evaporation!
Prepare a more suitable calibration range This solution makes possible calibration levels at lower concentrations. It is possible only if the LOQ is not already the lower limit of the calibration.
Prepare a new sample In the same conditions, the result will be identical!
A calibration blank…
must consist of water Warning: the sample is not necessarily aqueous.
is the diluent used to prepare the calibration range Yes, because it is the diluent, present in large quantities, which may contain impurities.
can be used a blank (reference) measurement Allows To check that the organic solvent and the water used to prepare the calibration range do not contain any impurities.
is most often useless In general, the solvents, water and reference standards used for quantitative analysis are very pure and the calibration blank is not necessary
A stock solution taken out of the refrigerator (at 5°C) and used immediately to prepare a calibration range at room temperature...
provides better accuracy No, because the volume taken is wrong!
leads to false volume sampling to wait until the solution is at the temperature of the experiment, often room temperature, before sampling precise volumes. When the molecules of a liquid are subjected to a rise in temperature (e.g. T°C from the refrigerator to room temperature), they accumulate energy and the Brownian motion increases. This results in an expansion of the volume => coefficient of thermal expansion α, specific for each solvent.
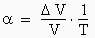
may present a false concentration Indeed, because in addition to the problem of false volume sampling seen above, it is possible that the solubility of the analyte contained in the stock solution is lower at 5°C than at room temperature. A part (invisible to the eye) may have precipitated, thus changing the concentration of the stock solution.
cannot be collected by pipette On the contrary, this solution can be collected and the two mistakes stated above will distort the result.
The internal standard…
must be initially present in the studied sample. Absolutely not, otherwise it will not be possible to know the initial concentration of the analyte.
must have a structure similar to that of the compound to be assayed Indeed, its physicochemical characteristics must be similar (see the "A little theory" tab of this sheet)
must always be added at the same concentration in the samples and levels of the calibration range (see the "A little theory" tab of this sheet)
Internal calibration…
eliminates the injection volume in gas chromatography. Indeed, it is difficult to inject a small volume (e.g. 1 µL) precisely. Therefore, the signal and concentration of the analyte are related to those of the internal standard in the construction of the calibration line, which makes it possible to dispense with the injection volume.
generally improves repeatability or reproducibility As the injection volume is released (case of manual injection) repeatability and reproducibility will be improved (the precision is improved). In addition, with an internal standard, it is possible to check the response of the device or to make sure that the automatic injector is working correctly (constant signal from the internal standard)
does not make it possible to ensure the correct functioning of an automatic injector On the contrary, it is possible to follow the signal of the internal standard which is always added at the same concentration in the solutions to be assayed.
is easier to implement than external calibration In fact no, because it is necessary to find an internal standard which has physicochemical characteristics close to those of the analyte, and an internal standard stock solution must be prepared and added at the same concentration to each solution to be assayed.
Calibration by standard additions…
should be used if there is a matrix effect Yes, this is even the advantage of this calibration method
is less accurate than the external calibration No, provided that there is no matrix effect
cannot make use of an internal standard On the contrary, it is possible, and when this is the case the signal and the added amount of analyte are related to those of the internal standard.
is onerous and expensive because it is like performing a calibration for each sample It is indeed necessary to add a known quantity of standard to each measurement for a given sample.
From the calibration line obtained by standard additions (y = ax + b), the concentration of the compound to be assayed is obtained for
x = 0
y = 0 Indeed the line is extended up to the x-axis. (See the " Illustrating Theory" tab of this sheet)
x and y = 0




