Mass spectrometer
As opposed to other spectrometry detectors, the mass spectrometer (often written MS) does not analyze the analytes according to their characteristics in reaction to light, but according to their molecular mass.
To do this, the mass spectrometry needs to generate ions that will then be analyzed according to the ratio between molecular mass (expressed in Dalton or Da) and the charge of the generated ion. This ratio is conventionally called m/z.
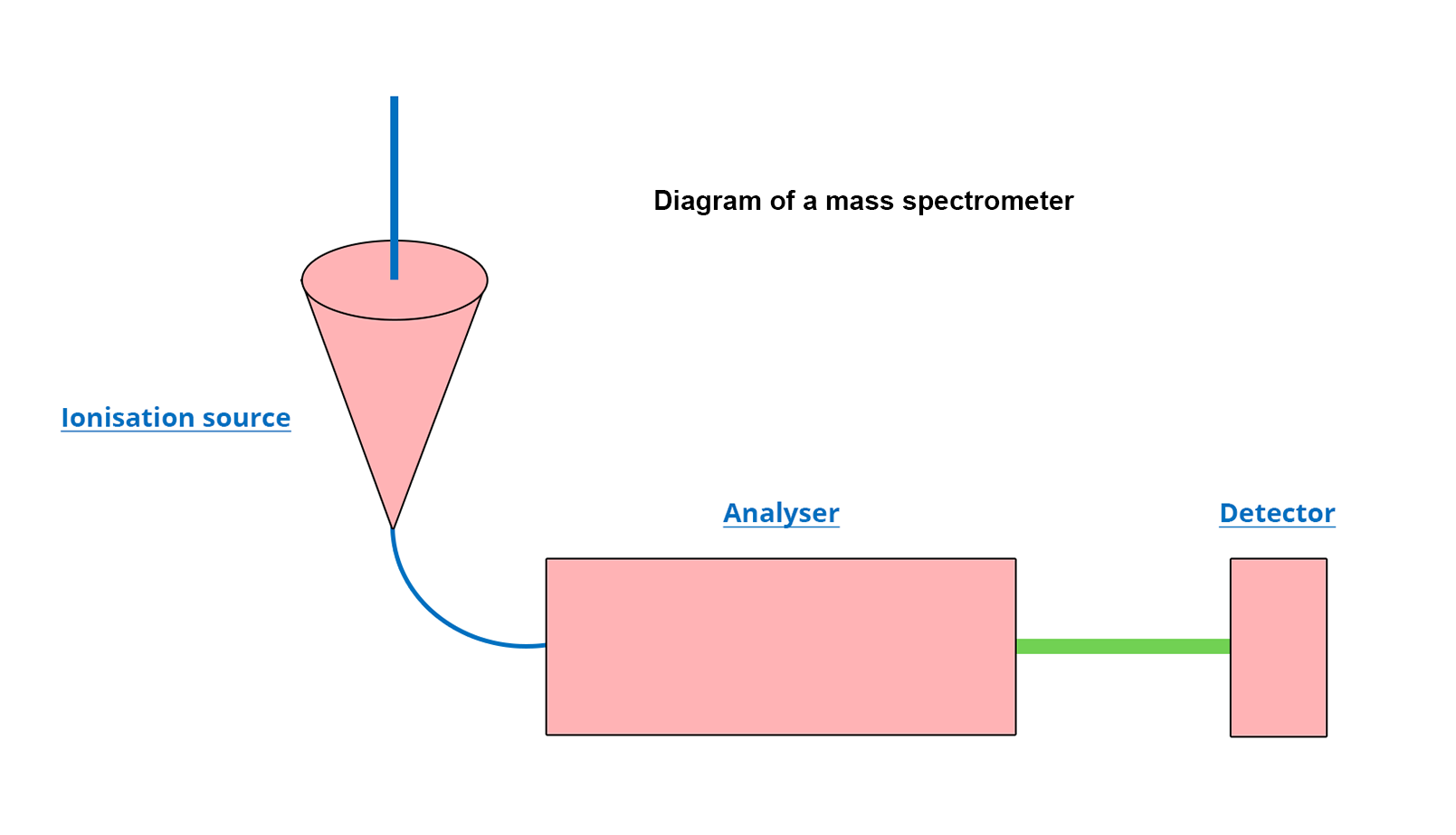
All mass spectrometers are made of three compartments visible in the diagram below (for additional description, click on the module):
The ionization source
When HPLC is coupled with a mass spectrometer (HPLC-MS), the ionization source has two major functions:
- To generate ions from analytes that have eluted from the column
- To evaporate the liquid mobile phase that could highly disturb the analysis of the generated ions.
Positioning the ionization source at 90° to the analyzer makes it possible to eliminate the analytes that would not have been ionized. The ions are directed towards the analyzer by means of an electric field generated by the voltage difference.
There are several possible ionization sources for HPLC-MS; the most widely used is electrospray ionization (ESI) which aims to form a spray of the mobile phase – this spray is made of ion-charged droplets due to the very high electric potential that exists between the ionization source and the inlet of the analyzer. The passage of a counter-current inert gas evaporates the mobile phase, ending up with ions at the source exit. These ionizing conditions being very mild, this generates little or no molecular fragmentation.
The analyzer
In MS, the analyzer separates the different ions generated by the ionization source according to their m/z ratio.
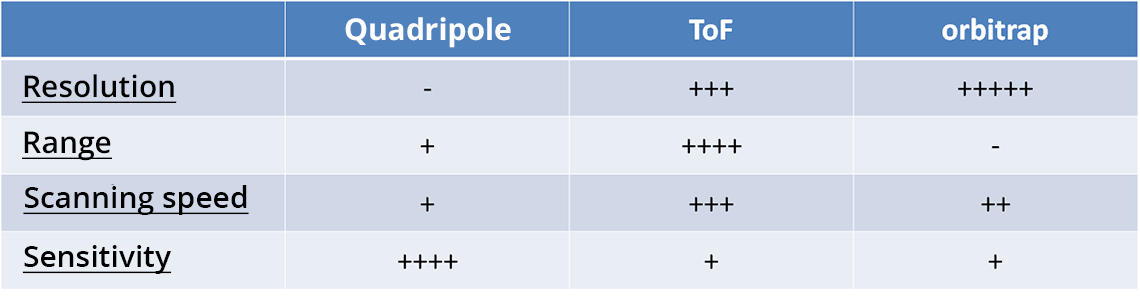
Although there are several analyzer principles and technologies in mass spectrometry, two fundamental parameters essentially come into play: molecule inertia (function of m) and ion electromagnetism (function of z). The most commonly employed are the quadrupole (Q), the time of flight (or ToF) and the orbitrap.
The important characteristics of an analyzer are:
- The resolution: apacity to differentiate between two analytes with very similar m/z ratios
- The m/z range: the more considerable it is, the more the analyzer will be able to work on a variety of molecules
- The scanning speed: parameter that evaluates the speed at which the m/z ratio can be changed during an analysis in order to detect several analytes simultaneously
- The sensitivity: the higher the sensitivity, the better the analyzer will perform, even for very low concentrations
Each type of analyzer has strengths and weaknesses concerning its characteristics. The choice is made according to what is to be measured.
The detector
The detector is the last step of the MS analysis. Once the ions have been generated and then separated in the analyzer, they must be detected (counted) in order to convert the signal.
As with the ionization source or the analyzer, there are several types of detectors.
In MS the historical detector is the photoelectrical plate. However, this detector has low sensitivity and does not allow for a quick data analysis. Recently, the preferred detector in MS is the electron multiplier. This detector consists of a lead-doped glass cone (Dynode) where the ions exiting the analyzer will ricochet, picking up new ions and thus amplifying the signal. This detector offers a very good sensitivity thanks to the signal amplification, but it loses in precision.