The column: main stage of the retention
In HPLC, the retention and separation of compounds depends on the stationary phase (the column) and mobile phase (the solvent mixture). The most commonly used chromatographic method is currently the so-called reversed-phase partition chromatography (non-polar stationary phase and polar mobile phase).
The most commonly used stationary phase in reversed-phase partition chromatography is composed of C18-bonded silica which offers numerous advantages but also has its own limits.
The C18-bonded silica: an almost universal stationary phase…
Octadecyl-bonded silica (or C18-bonded silica) is always obtained by chemical bonding of pure silica particles (Si-OH). The binding groups (alkyl-groups of octadecane: alkane of 18 carbons - C18) are covalently bonded to the silica. The bonding rate may vary with column manufacturers. A ‘finishing treatment’ (end-capping) is carried out most of the time to minimize the presence of residual silanols that may retain polar compounds.
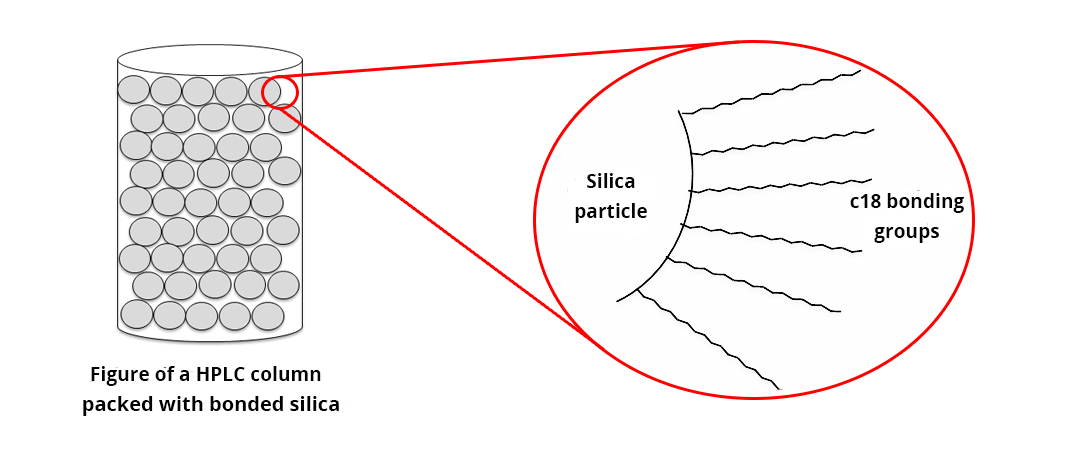
Since octadecane is a very non-polar alkane, the stationary phase of C18-bonded silica will have a very high affinity with low polar or non-polar compounds. To optimize the retention of these compounds, the mobile phase used is often aqueous in order to strengthen the hydrophobic bonds between the column and the studied compounds (cf. The pump).
Advantages and limitations of reversed-phase chromatography on C18 columns
| Advantages | Limitations |
|---|---|
| Allows separation of polar and non-polar compounds | Poor retention for very polar compounds |
| Column is resistant to extreme conditions (pH: 2 – 8 or 11, use of additives) | Impossible to separate isomers |
| Less organic solvent is used: mobile phase mainly aqueous | Impossible to use a 100% aqueous phase (risk of permanent deterioration of the C18 structure) |
| Highly resistant column over time |
The HPLC column
Unlike GC columns, the columns used in HPLC are not hollow, but filled with stationary phase particles. These particles are generally made of silica that can then be surface-bonded with different polar or non-polar groups (such as C18-bonded silica).
HPLC columns are generally characterized by three varying physical quantities:
- The column length: generally varies between 5 cm and 25 cm
- The internal diameter: generally varies between 4.6 mm and 1 mm
- The particle size varies between 2 µm and 5 µm (smaller particles are possible with the UHPLC)
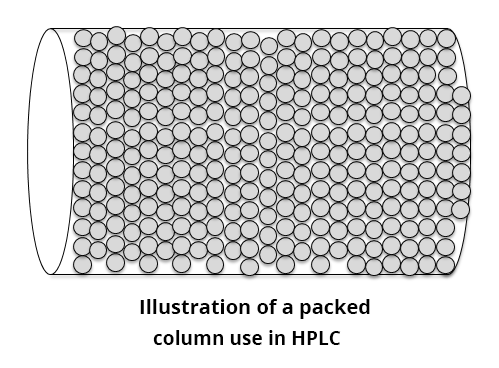

The use of a column packed with particles improves the surface exchange between mobile phase and stationary phase for short columns. However, the presence of particles in the µm range creates very high pressures at the column inlet. In conventional HPLC, the pressure can reach 415 bars, which is equivalent to the pressure encountered on the ocean floor. For UHPLC, the pressure at the head of the column can reach 1500 bars, i.e. a pressure greater than the pressure measured in the deepest fault in the oceans (12 km deep). By way of comparison, the pressure of domestic running water networks is around 3 bars.
The thermostatically controlled column compartment
Although it may seem unimportant, having a thermostatically controlled compartment for the chromatographic column plays an important role in the retention or elution of compounds.
Indeed, a temperature variation in the column could change the retention time of the compounds and interfere with the analysis.
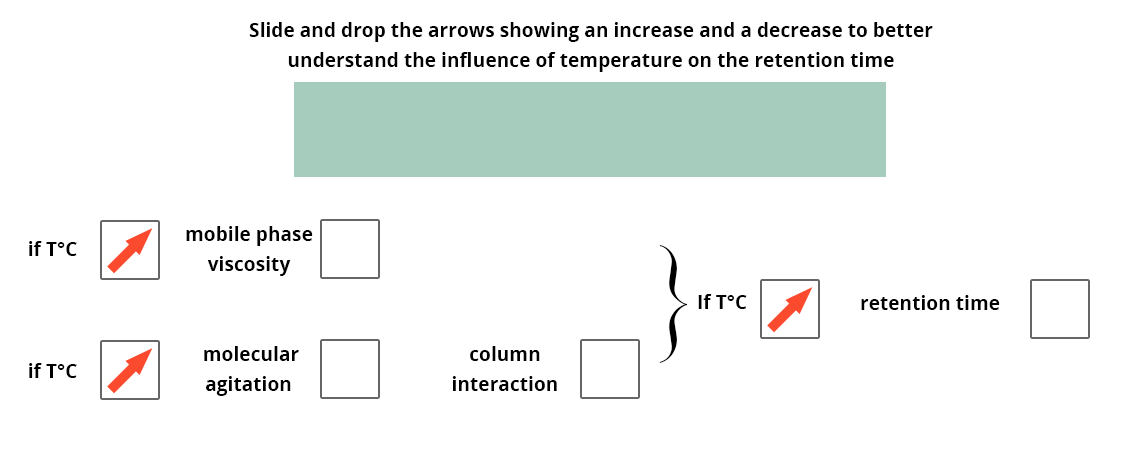




Failure to maintain the column temperature will cause a variation in the retention times of the compounds between winter and summer if the room is not air-conditioned.
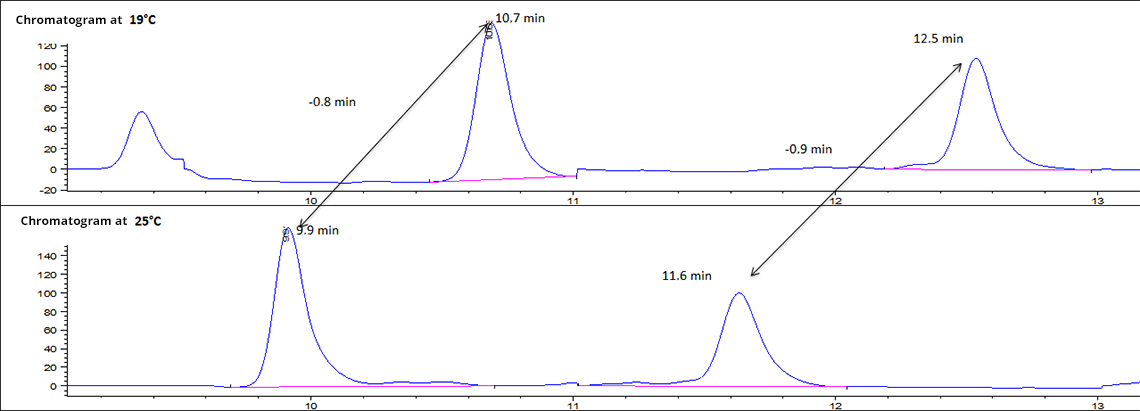
The chromatogram below allows you to see the differences in retention of the analyzed compounds between 19°C (winter in a heated room), 25°C (temperate summer). Based on the retention time, it is impossible to analyze the compounds in winter and in summer because they are impacted by the temperature and the analysis is not reproducible throughout the year.
In the vast majority of cases, the thermostatically controlled compartment (also known as the column oven) is used to ensure temperature stability in both winter and summer. Because of this, the programmed temperature is often higher than 30°C because older ovens are unable to refrigerate, meaning the temperature has to be higher than the outside temperature in summer.
More recently, column oven technology has evolved and column ovens are capable of heating or cooling the column. This capability has allowed for new applications, particularly using low-temperature separations (10°C or below), to maximize the retention of poorly retained compounds in the column.





