Van der Waals interactions
There are 3 types of Van der Waals forces:
- Orientation forces (or Keesom forces)
- Induction forces (or Debye forces)
- Dispersion forces (or London forces)
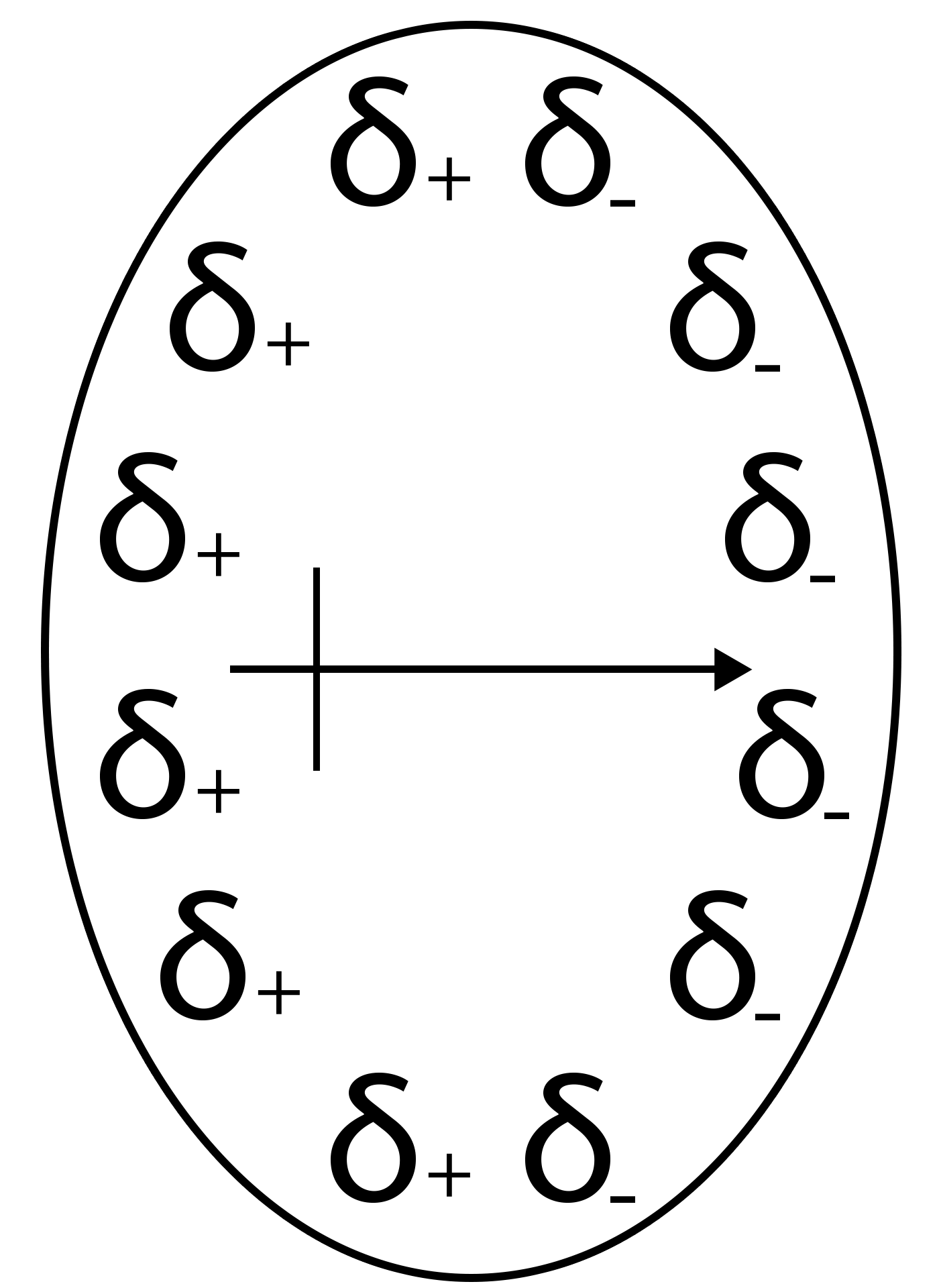
Orientation forces
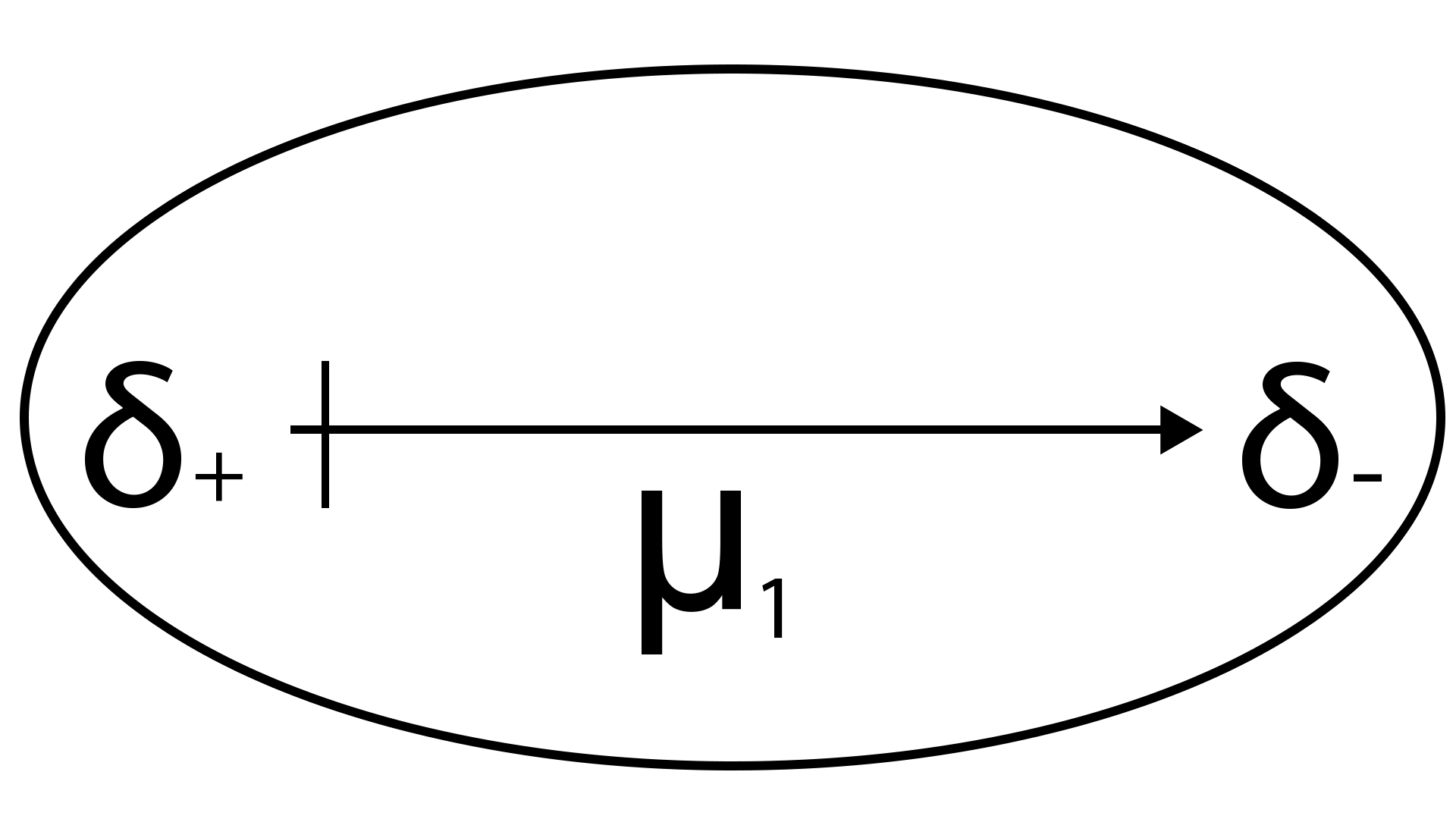
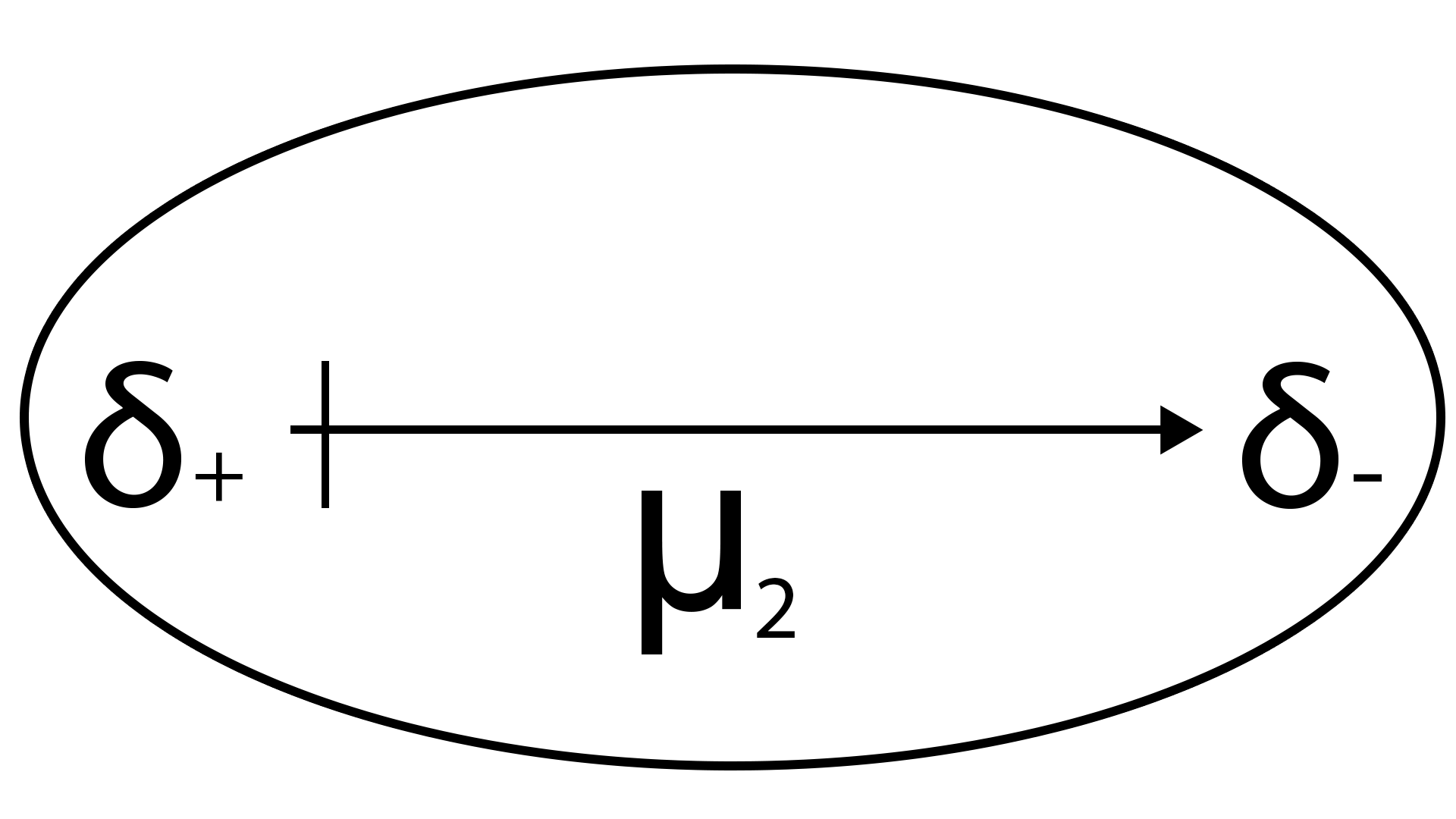
r: distance between the 2 dipoles
T: temperature
Induction forces

Dispersion forces