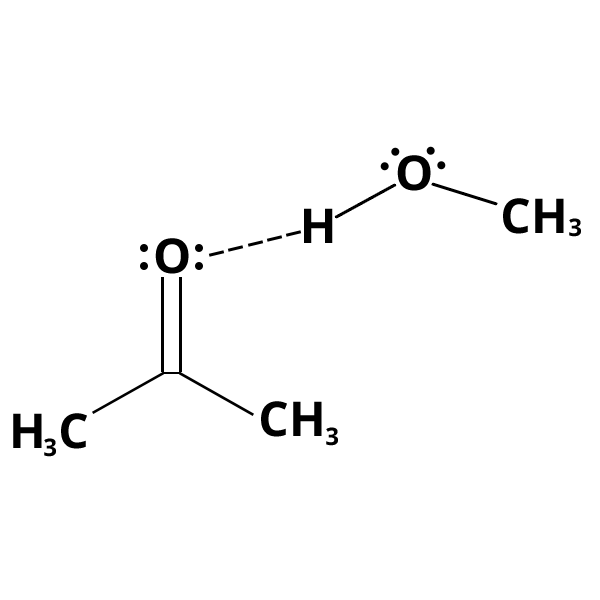
Hydrogen bonding
These specific forces occur between compounds with functional groups in which a hydrogen atom is bonded to a small electronegative atom (cf. example).
An inter- (or intra-) molecular hydrogen bond is formed through the X–H and Y groups:
X-H: this group provides the proton of the hydrogen bond (proton donor group)
Y: electron donor group (presence of a free electron doublet)
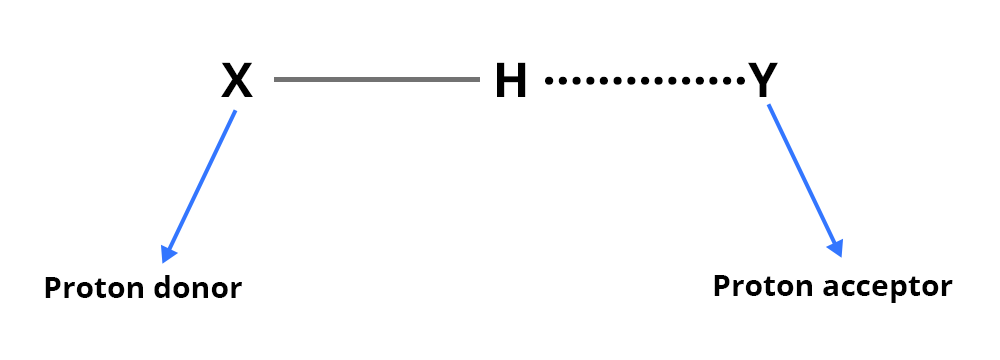
This energy is of the order of 25 to 40 kJ/mol.